The periodic table and classification of
elements
1- The elements are arranged in an ascending order according to their atomic number.
2- As we know from the previous years that the periodic table is divided into four main
blocks:
1- S – block elements 2- P – block elements
3- d – block elements 4- f – block elements
3- The elements of S and P blocks are named by representative elements and they
include all A-groups. While the d-block elements are called main transition elements
and they include all B-groups . f-block elements are called inner-transition elements.
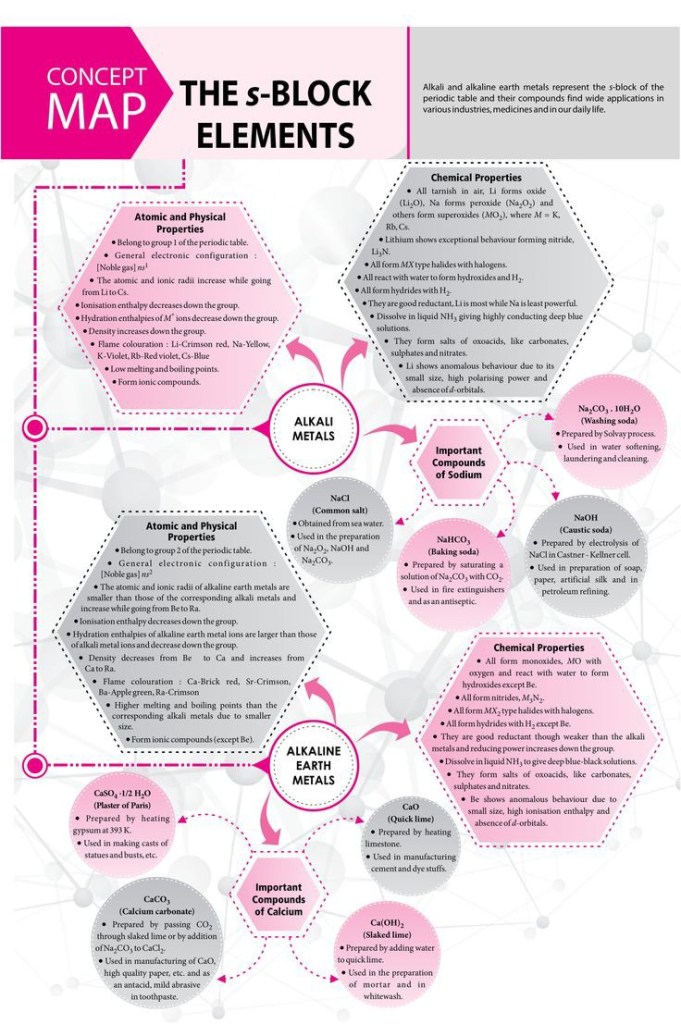
a) S-block elements
1-They are located in the left-hand block of the periodic table.
2- It consists of two groups: Group 1(alkali metals) as there is one electron in s-orbital
and electronic configuration of it is ns1
and Group 2 (alkaline earth metals) as there is
two electrons in s-orbital and electronic configuration of it is ns2
.
3- They contains two groups only as the S – sublevel consists of one orbital which is
filled with two electrons only .
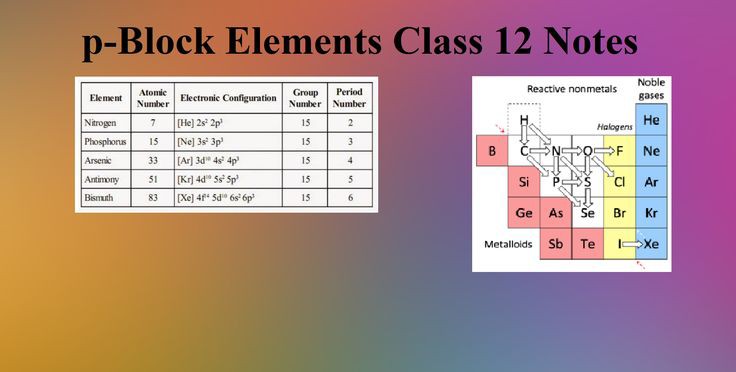
b) p-block elements
1-they are located in the right-hand block of the periodic table.
2- they are six groups, starts from the 13th group and goes till
the 18th group(zero group) in the periodic table.
3- they contain six groups as the P – sublevel consists of three orbitals which filled
with six electrons
c) d-block elements
1-They are located in the middle of the periodic table.
2-They are 10 groups, where its elements having electrons (1 to 10), and they occupy
columns 3 to 12, seven of which belongs to the B group and three belong to group 8.
3-They contains ten groups, as the d – sublevel consists of five orbitals which are
filled with ten electrons.
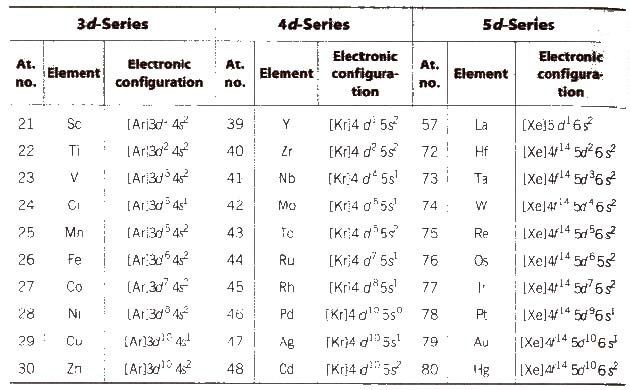
- 1- First transition series
1-they include elements in which 3d sublevel is filled, located in period 4 .
2-They start from scandium (Sc) and ends with zinc (Zn) .
- 2- Second transition elements
1- they include elements in which 4d sublevel is filled, located in period 5.
2- they start from yttrium (Y) to cadmium (Cd)
- 3-Third transition series
1- They include elements in which 5d sublevel is filled, located in period 6 .
2-They start from lanthanum (La) to mercury (Hg).